Quality Assurance
Samir G. Khoury, Ph.D., P.G.
Course Outline
This course introduces and describes the principles of Quality Assurance (QA) and the elements of a formal Quality Assurance Program. The concepts presented are applicable to projects and activities ranging from the design and construction of large and small buildings and structures, systems, subsystems, the manufacturing of components or complete products to providing professional services such as the implementation of site assessments and environmental and hazardous waste investigations. As such, the principles and concepts presented here are equally useful to all types of engineers, constructors, architects, contractors, building inspectors, surveyors and environmental and geological scientists. The payback of a well designed QA program includes:
- delivering higher quality products and services,
- reducing the time and money wasted in re-performing activities or correcting errors,
- enhancing your professional reputation for delivering quality work on schedule and within budget,
- minimizing crisis management and the associated stress,
- potential reduction in loss time accidents or injuries, and
- enhanced protection from the damaging effects of lawsuits and litigation.
This course addresses the 18 QA categories (criteria) that are formally identified in the Code of Federal Regulations. For each of the 18 criteria, the following questions are discussed: "What does this criterion address?", "Why is this criterion important?", and "How is this criterion implemented?". The following is the course outline:
- Introduction
- Components
of a Formal QA Program
- Criterion #1: Organization
- Criterion #2: Quality Assurance Program
- Criterion #3: Design Controls
- Criterion #4: Procurement Document Control
- Criterion #5: Instructions, Procedures and Drawings
- Criterion #6: Document Control
- Criterion #7: Control of Purchased Material, Equipment and Services
- Criterion #8: Identification and Control of Materials, Parts and Components
- Criterion #9: Control of Processes
- Criterion #10: Inspection
- Criterion #11: Test Control
- Criterion #12: Control of Measuring and Test Equipment
- Criterion #13: Handling, Storage and Shipping
- Criterion #14: Inspection, Test and Operating Status
- Criterion #15: Nonconforming Materials, Parts or Components
- Criterion #16: Corrective Action
- Criterion #17: Quality Assurance Records
- Criterion #18: Audits, Surveillance and Managerial Controls
- Conclusion
This course includes
a multiple choice quiz at the end, which is designed to enhance the understanding
of the course materials.
Learning Objective
At
the end of this course, you will understand the purpose and benefits of a formal
Quality Assurance Program, and be familiar with the 18 components of such a
program. For each of the 18 components, you will learn: 1) what aspect of design,
construction, manufacture or site studies the component addresses, 2) why this
aspect is an important part of a Quality Assurance Program, and 3) how this
component is actually implemented. You should then be able to evaluate, for
your own projects or activities, which of these components are applicable, to
what extent or degree the component should be applied, and what benefits you
might realize if the component is properly implemented.
Course Content
- Plan on doing it right.
- Do it right the first time.
- Document what you did.
Item 1 should be a no-brainer. Hopefully anyone intending to design and/or build a structure, manufacture a product, conduct site investigations or do environmental clean-up plans to do it right.
Item 2, do it right the first time, is an important management issue. Doing it over again after a failure usually costs money, erodes goodwill and damages your reputation, and may open the door to lawsuits and liabilities ranging from breach of contract, delay of schedule, or, even worse, injury or loss of life. Implementing a properly designed QA program should allow you to identify problems early and take appropriate corrective action before the problem grows to critical proportions. Finding and correcting problems early is the next best thing to not having problems in the first place.
Item 3 is probably the most overlooked yet critical concept. Documentation takes time and effort, and therefore costs money. However, it provides a critical paper (or electronic) trail that allows you to reconstruct what actually happened, and not rely solely on the often unreliable recollections and memories of the people involved. Furthermore, it should be remembered that even if you did everything right and something happens later that is out of your control, somebody may still point a finger at you. Having formal QA procedures in place and documentation that your procedures were implemented may well save you from serious expenses and legal distractions down the road.

Components of a Formal QA Program
The Federal Regulations that were originally developed to control the design, construction and operation of nuclear power plants identified a series of 18 "criteria" or components that need to be addressed in developing a comprehensive quality assurance program. The 18 criteria are now formally published in Title 10, Part 50, Appendix B of the Code of Federal Regulations (10 CFR 50, Appendix B). The concepts formalized in these regulations provide an excellent framework within which to develop a quality assurance program for virtually any type of project. The same principles apply to the engineering design and construction of structures, the manufacturing of products, providing professional services, and the implementation of site assessments and environmental projects such as hazardous waste clean-up. The 18 criteria identified in the federal regulations are:
- Organization
- Quality Assurance Program
- Design Controls
- Procurement Document Control
- Instructions, Procedures and Drawings
- Document Control
- Control of Purchased Material, Equipment and Services
- Identification and Control of Materials, Parts and Components
- Control of Processes
- Inspection
- Test Control
- Control of Measuring and Test Equipment
- Handling Storage and Shipping
- Inspection, Test and Operating Status
- Nonconforming Materials, Parts or Components
- Corrective Action
- Quality Assurance Records
- Audits Surveillance and Managerial Controls
Each of these 18 criteria is addressed below. For each criterion, three questions are answered: "What does this criterion address?", "Why is this criterion important?", and "How is this criterion implemented?"
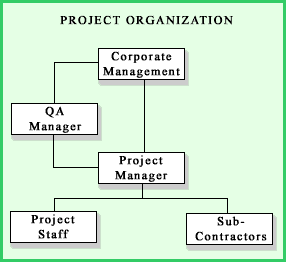
Criterion #1: Organization
What?
This criterion addresses the organization of the QA function including personnel and their responsibilities and authority. The QA organization includes not only your staff, but also staff from suppliers and contractors.Why?
The most critical component of any QA program is people. Everyone involved in the QA process needs to know exactly what their duties and responsibilities are, who they report to on QA matters, and what level of authority they have to identify, report, and correct problems. If things go wrong, you don't want everybody claiming "that wasn't my job!".How?
An organization chart is developed for every project. The position and role of QA personnel should be clearly delineated and the persons performing QA functions should have sufficient authority and freedom to identify problems and initiate corrective action in a timely manner. To maintain his/her independence from the project, the QA manager should report directly to corporate management.If your organization uses outside suppliers and/or contractors, the QA chain of command must be extended into their operations. The QA process involves everyone and transcends corporate divisions and organizational boundaries. Names of people should be included on the organization chart. When staffing changes are made, the chart should be updated.
For each position on the organization chart, a functional description should be developed outlining the individual's responsibilities and authority. For example, does an individual have the authority to reject a shipment of incorrect materials from a supplier, or does he/she have to contact their supervisor first?
Criterion #2: Quality Assurance Program
What?
This criterion reflects the need to think through all the elements of the overall QA program and to document: 1) the aspects of your designs, structures, products, services, or activities that fall under the QA program and 2) the details of QA program implementation. This document is the roadmap to guide you from concept to reality.Why?
Without a roadmap, the QA program is just an idea. Developing and formalizing the roadmap requires you to think through what you want the QA program to accomplish and to define the steps required to reach your objectives.How?
A Quality Assurance Program Plan is written prior to initiating the work. The plan should systematically address each of the 18 criteria discussed in this course, and has several major objectives:
- To identify
which aspects of your organization or project should fall under the QA program.
For example, the QA program may apply to the manufacture of one or more products,
or apply to an environmental site clean up or construction projects. It probably
won't apply to the human resources department.
- To assess which
of the 18 criteria apply. For example, there are criteria dealing with procurement
documents. If your organization or project does not procure goods or services,
this criterion is not applicable.
- For each applicable
criterion, to develop the implementation details, preferably before the activity
starts. This aspect usually requires defining a set of procedures. It may
also require designing forms to be used in tracking and documenting certain
activities.
- To address training needs for those people involved in the QA program. Determine who needs training and what do they need to learn to implement the program as designed?
Everyone involved should read, comment on, and hopefully, improve the Quality Assurance Program Plan . Also, with time and experience, procedures and approaches will undoubtedly change. When they do, the original program plan should be modified and updated by adding appendices outlining the changes. Once a year, look over what was written and see if you are still on track. What worked and what didn't? Think of QA as a process, not a goal.

Criterion #3: Design Controls
What?
For this purpose the term "design" is broadly defined as:
- Specifications,
plans, drawings, blueprints, CAD files, and similar items
- Information
contained in written documents related to the design of products, structures
or activities
- Research and development data pertinent to the design
This criterion addresses the need to verify the information contained in the plans, drawings, specifications, and related documents pertaining to the design of products, structures or activities.
Why?
Before products are manufactured, buildings or structures constructed, or site investigation activities performed in the field, a plan is developed. This plan consists of all the relevant design drawings, instructions and supporting documentation. If there are mistakes in the plans or instructions, then the outcome will not be right.The design controls criterion is intended to catch two types of design errors:
- Conceptual errors;
such as confirming that the structure, product or activity will in fact be
suitable and adequate for its intended purpose. This is termed "design verification",
and
- Implementation errors, such as confirming that numerical calculations were done correctly, the accuracy of data input into computer programs, and/or the appropriateness of the computer programs used. This is termed "design checking".
By having design controls in place up front, the chances of failure at the end are minimized. Obviously for large structures like dams, power plants, or buildings where failure could cause loss of life, such controls are critical. However, even for manufacturing, small construction jobs, site investigations, or environmental clean-up, this up-front work can potentially save money, help maintain credibility and reputation, and minimize liability.
How?
The QA Program Plan includes a design control plan, which addresses:
- The procedures
to ensure design verification. These procedures can be as simple as review
by other qualified staff, or as complex as destructive testing,
- The procedures
to ensure design checking, such as independent calculations, use of alternative
calculation methods, checking data entry files, and/or ensuring that the correct
computer programs are used,
- The identification
of people responsible for design verification and design checking. These individuals
should not be the original designers or their immediate supervisors. Design
verification should be done by individuals at least as skilled or experienced
as the original designer. Design checking, however, can be done by less skilled
workers, as long as the procedures are thorough and detailed, and
- The process, by which any design changes, including changes in the field, are reviewed and documented. Such changes should be subject to the same design controls as the original design.

Criterion #4: Procurement Document Control
What?
Procurement documents are the basis for obtaining goods and services from suppliers and contractors. Some supplies or contract work may be simple and not worth addressing in a QA plan, Other supplies or contract work may directly affect your ability to design, manufacture, construct and/or complete a project successfully. In these cases, it is important that procurement documents are planned, reviewed, released and distributed with care.Why?
If you are using goods and services from suppliers and contractors that affect the quality of your final product, structure or service, then you have a vested interest in the quality of those goods or work products. Having an excellent in-house QA program, but no control over the quality of critical materials, components, equipment, work products or test results from your suppliers or contractors reduces the effectiveness of your program.How?
Critical procurement processes are designed to address the following objectives:
- Identification
of the people responsible for the procurement documents,
- Definition of
a process for internal review, approval and issuance of procurement documents,
- Identification
of acceptance and rejection criteria for materials or work products,
- Specification
of the QA programs that suppliers and contractors must implement, commensurate
with the importance of the materials or work products they contribute to your
final product, structure or service, and
- Evaluation of potential suppliers and contractors to ensure they can meet your procurement requirements.
In addition to this plan, the procurement documents should also identify which records must be maintained by the supplier or contractor and which should be delivered to you before their material or work product is used. You should also require that the supplier or contractor permits you to visit their facilities and access records pertaining to your orders.

Criterion #5: Instructions, Procedures and Drawings
What?
This criterion addresses the need to ensure that activities affecting quality are performed in accordance with instructions, procedures and drawings. It also addresses the need to develop acceptance and rejection criteria.Why?
There are three primary reasons to maintain planned and approved instructions, procedures and drawings for performance of quality activities:
- To ensure the
activities are performed in a consistent and specific way or sequence,
- To provide criteria
to base an independent verification that the activity was performed correctly,
and
- To document the tools, methods and results of the activities for future review and evaluation.
How?
For each process affecting quality, sets of procedures are developed to guide the responsible staff in the testing and/or evaluation of work products. As part of these procedures, metrics should be specified as to what is acceptable and what is cause for rejection. Such metrics can be as simple as dimensions and tolerances for manufactured parts, to more complex procedures such as comparing test results of duplicate samples sent to laboratories.Criterion #6: Document Control
What?
Document control is the process of 1) ensuring that documents used for the quality assurance process have been reviewed and approved by qualified individuals, and 2) ensuring that all persons needing these documents have access to the most current copies.Why?
The procedures and drawings to be used for the QA process form the foundation upon which the QA program is based. Therefore, before QA activities start, it is critical that these instructional documents are properly prepared, reviewed and approved. It is also important that any updates and changes to these documents be distributed to the staff responsible for their implementation.How?
The first step in implementing this criterion is to identify the types of documents that should be controlled. For example, a document that describes the details of a testing procedure and the acceptance and rejection criteria would be identified as a controlled document.Once the documents to be controlled have been identified, three activities are initiated:
- The original
documents are reviewed and approved by the appropriate staff prior to the
start of the activity. This review and approval process can be documented,
for example, by signoffs on a cover page,
- Any changes
made to the original documents should be reviewed and approved by the same
people who were responsible for the original review and approval, unless formally
delegated to others, and
- A master list should be set up and maintained to track the latest dates and revision numbers of controlled documents and drawings. This list should include the names of all personnel for distribution of the documents, and should also track that each of them has been sent and has received the latest revisions.
Criterion #7: Control of Purchased Material, Equipment and Services
What?
Procurement documents are used to order goods and services from suppliers. This criterion reflects the need to ensure that goods and services provided by suppliers and contractors actually meet the requirements spelled out in the procurement documents.Why?
Without these controls, it is not possible for you to know if the goods and services purchased actually meet specifications.How?
There are six steps designed to control the process of purchasing material, equipment and services, as follows:
- Evaluation of the supplier prior to order or contract award,
- Review of evidence that the contractor or supplier can meet your requirements,
- Requiring documentation that your specifications have in fact been met prior to shipment,
- Inspection of materials or equipment at the suppliers facilities, prior to shipment,
- Documentation that received materials and equipment do meet the specifications prior to use, and
- Periodic assessment of how effectively your contractors and suppliers control quality.

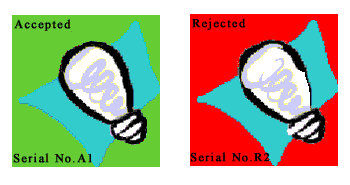
Criterion #8: Identification and Control of Materials, Parts and Components
What?
This criterion addresses the need to identify and control the use of materials, parts and components to minimize the possibility that incorrect or defective items are used.Why?
It is evident that defective parts and components should not be used. However, even if parts or materials (for example, chemicals or solvents) are of high quality, if they are not used as intended, the final product may be defective or unusable.How?
There are three components to the identification and control process:
- All items should
be identified by part numbers, serial numbers or other appropriate markings,
- Identification
should be maintained throughout the use of an item, either directly on the
item or through a tracking system, and
- The method of identification or tracking should not affect the function or quality of the item.
Criterion #9: Control of Processes
What?
This criterion addresses the implementation of processes that affect the quality of items or services. Examples of such processes include the execution of tasks, the production of goods, the implementation of services, or verification of quality.Why?
When procedures are developed for processes that affect quality, assurance must be provided that these procedures are implemented properly by qualified individuals. Otherwise, they are of little use or value.How?
Processes that affect quality of goods or services are documented by instructions, procedures, drawings, checklists, or similar techniques. The processes themselves should be performed by qualified personnel using the documented procedures. Training of personnel should be documented and kept current.Criterion #10: Inspection
What?
The primary purpose of the inspection activities is to accept or reject completed work.Why?
Inspection is critical to verify that the activities implemented to assure quality have been properly performed.How?
Inspection can be accomplished in one of two ways: 1) by direct inspection of work products or processes and/or 2) by monitoring the activities that create the products or processes. The monitoring approach is used when direct inspection is impossible or disadvantageous. In some cases, both direct inspection and monitoring are used when either approach alone is considered inadequate.The components of both the direct inspection and monitoring approach are:
- Ensuring that
the inspection is performed by qualified individuals, other than those who
performed the work,
- Keeping the
qualifications or certifications of inspectors current,
- Ensuring that
the procedures required for the inspection are made available before the inspections
are performed,
- Scheduling inspections
for each work operation as necessary,
- Ensuring that
modified, replaced, repaired or reworked items are inspected in accordance
with the original inspection procedures, and
- Identifying mandatory inspection hold-points where work will not proceed unless authorized.

Criterion #11: Test Control
What?
A test is defined as an operation performed to resolve an uncertainty. A test may be conducted to determine if an item or service is acceptable, or to acquire additional information.Why?
Without testing, it is not possible to verify that parts, components or finished products will function as designed.How?
The testing component of a quality assurance program includes the documentation and procedures to ensure that:
- A description
of the test exists, including its purpose and how it will be used to understand
the performance of a system, component or structure,
- Written test
procedures exist,
- Tests will be
performed by qualified and properly trained individuals,
- All prerequisites
for a given test have been met,
- Adequate test
instrumentation and equipment are available,
- The test is
performed under suitable conditions using adequate testing methods, and
- Test results are documented and stored as QA records

Criterion #12: Control of Measuring and Test Equipment
What?
This criterion addresses the requirement that all measurements affecting quality should be taken only with instruments, tools and gauges that are accurate and calibrated at pre-determined intervals to maintain accuracy within specified limits.Why?
Tests performed with equipment that is inaccurate, out of adjustment or not calibrated will provide faulty results. Therefore, any decisions based on these results will likely be invalid.How?
Procedures are developed to control measuring and test equipment that:
- Describe the
control and maintenance procedures for each piece of test equipment, including
the frequency of calibration,
- Describe the
inspection and monitoring activities needed to ensure these procedures are
completed,
- Ensure that
each piece of test equipment is labeled, tagged or otherwise documented to
indicate when the next calibration is due,
- Track the calibration
history of each piece of equipment, including drift and other characteristics
so that the performance history of the equipment can be evaluated,
- Ensure that
the reference calibrations are done to recognized standards, and
- Retain documentation of calibrations in the project files.

Criterion #13: Handling, Storage and Shipping
What?
This criterion addresses issues concerning the deterioration or damage to materials or equipment during handling, storage and shipping.Why?
Allowing materials or equipment to deteriorate or be damaged during transit or storage will likely affect the quality of the final products created using such material, equipment or supplies.How?
Procedures are developed for handling, storage and shipping of equipment and/or supplies that are critical to quality of your products or services. An example would be the storage of chemicals and solvents in areas that maintain the materials within the range of temperatures and humidity that will prevent deterioration. Other examples might include specifying cleaning procedures for field sampling and testing equipment, or the type of shipping containers to be used to protect delicate samples. Part of this criterion addresses the need for qualified and trained individuals to manage the handling, cleaning, packaging, shipping, preservation and storage of materials in accordance with written work instructions.

Criterion #14: Inspection, Test and Operating Status
What?
This criterion addresses the need to track which samples, structures, systems and components have been tested and their status.Why?
The purpose of this criterion is to prevent the inadvertent use of unacceptable materials or parts.How?
Procedures are developed to stamp, tag or otherwise label materials or parts that have been tested and document the results of the test. Those materials and parts that have satisfactorily passed the required inspections and tests should be clearly identified and stored separately to preclude the inadvertent bypassing of such inspections and tests.Criterion #15: Nonconforming Materials, Parts or Components
What?
This criterion addresses the need to control any materials, parts or components that are not acceptable to ensure that they are not used.Why?
If materials or parts are tested, and the tests indicate that the material or part is unacceptable, it needs to be removed to prevent its inadvertent use.How?
Procedures are developed to ensure that:
- Materials, parts
or components that do not conform to requirements are identified, documented,
and segregated to prevent inadvertent use,
- Organizations
affected by the non-conforming materials, either the suppliers or the end
users, are notified, and
- The non-conforming materials or parts are repaired, reworked, or disposed of in accordance with documented procedures.
Criterion #16: Corrective Action
What?
Corrective Action is defined as those measures that are taken to ensure that any conditions leading to failures, malfunctions or deficiencies are identified and corrected. In the case of significant conditions adverse to quality, the measures taken shall assure that the cause of the condition is determined and corrective action is taken in order to preclude repetition.Why?
If a process or procedure, a supplier or design is linked to an unacceptable level of rejection during inspection and testing, it only makes sense to identify the problem and fix it. Documenting the problem and how it was corrected provides a written record that makes reconstruction of the events much simpler even years after the fact. This record may prove invaluable in the face of litigation, and as part of a knowledge base within the organization that can save time and money down the road.How?
When a problem is identified, the right people are brought in to address and solve it. The problem may be with the original design, or may be related to faulty equipment or incorrect procedures. Each problem is addressed on a case-by-case basis. This process goes on in businesses and organizations every day, independent of whether or not they have a formal QA program. The Quality Assurance aspect of this process is the documentation of the problem and its corrective action. The documentation includes the identification of the problem, its probable cause, and the action taken to correct it. Documentation is generally forwarded to the appropriate level of management and stored in the project file.Criterion #17: Quality Assurance Records
What?
QA records furnish evidence that activities affecting quality have been properly performed.Why?
In the event of problems, disputes, or even lawsuits concerning your designs, buildings or structures, products, or services, the documentation of your QA activities, if properly completed, provides a critical historical record.How?
Most of the activities described in the earlier criteria have a documentation component. Such documentation includes:
- Operating logs,
- Results of reviews, tests, inspections, audits, monitoring, and analyses of materials,
- Qualifications of personnel,
- All the procedures developed to guide activities, and
- The acceptance certification of the various systems, parts and components that are used.
Inspection and test records should, at a minimum, identify the inspector, person who recorded the data, types of observations, results, and documentation of acceptance, rejection or deficiencies noted.
This criterion also addresses the need to establish requirements for:
- Retention of QA records, including formats (paper, electronic),
- Security of these records,
- Duration of retention, and
- Assigned responsibility.
QA records should be protected from destruction by fire, flooding, severe weather, insects and rodents, and protected from deterioration caused by extremes in temperature and humidity. Electronic records should have appropriate backup and protection procedures in place.
Criterion #18: Audits, Surveillance and Managerial Controls
What?
This criterion addresses the need to ensure that all aspects of the QA program are being properly implemented. By using well planned verification techniques, one is better able to demonstrate that design, construction, and operation activities conform to the requirements of the QA program.Why?
Without assurance that the QA program is actually being implemented, you have no assurance that the program is accomplishing your objectives. Real-time awareness of anomalies permits immediate analysis and correction of problems as they are identified.How?
There are several aspects of the audit and surveillance program that are addressed:
- External audits,
which are performed on your primary contractors and suppliers,
- Internal audits,
which are performed within your own organization,
- Planning and
scheduling of audits to ensure that they are initiated early enough to catch
problems before quality is seriously and irretrievably affected,
- Conducting the
audits in accordance with pre-defined procedures or checklists prepared by
qualified personnel not having direct responsibility in the area being audited,
and
- Documentation of the results of audits with review by management personnel, and, where necessary, documentation of follow-up actions taken, including re-audit of the deficient areas.
Conclusion
Quality Assurance (QA) comprises the planned and systematic actions that need to be taken in order to certify that a designed and constructed structure or a manufactured product will perform satisfactorily in service (10 CFR 50, Appendix B). In the case of studies requiring the collection and analysis of critical data, such as environmental site investigations, QA comprises the necessary actions needed to provide adequate confidence in the validity and integrity of the data obtained. The objectives of the QA program are generally accomplished by defining and tracking the steps taken to implement the project or activities in accordance with clearly defined and documented instructions and specifications, ensuring that staff have appropriate and adequate training, and implementing an inspection and monitoring program. For each project or activity, an evaluation of which of the 18 regulatory criteria are applicable, and to what degree the criterion should be implemented is well worth the time and effort involved. The payback of a well designed QA program includes:
- delivering higher quality products and services,
- reducing the time and money wasted in re-performing activities or correcting errors,
- enhancing your professional reputation for delivering quality work on schedule and within budget,
- minimizing crisis management and the associated stress,
- potential reduction in loss time accidents or injuries, and
- enhanced protection from the damaging effects of lawsuits and litigation.
Quality Assurance in PDF Format (629 KB).
![]() You may need to download Acrobat Reader to view and print the document.
You may need to download Acrobat Reader to view and print the document.
Once
you finish studying the
above course content,
you need to
take a quiz
to obtain the PDH credits.
DISCLAIMER: The materials contained in the online course are not intended as a representation or warranty on the part of PDHonline.com or any other person/organization named herein. The materials are for general information only. They are not a substitute for competent professional advice. Application of this information to a specific project should be reviewed by a registered professional engineer. Anyone making use of the information set forth herein does so at their own risk and assumes any and all resulting liability arising therefrom.
